What Is Relaxivity?
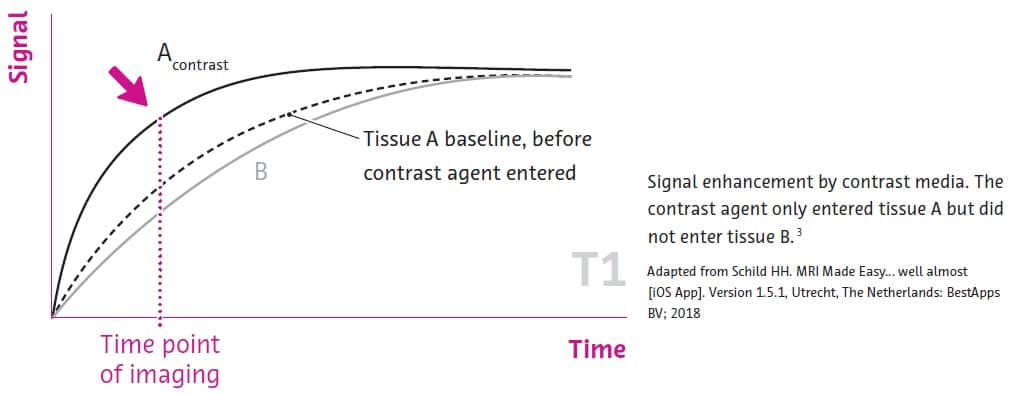
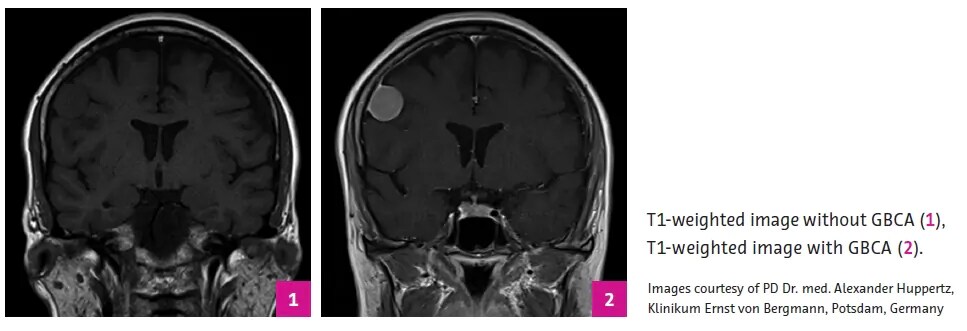
.
1 Balzer T. in , Reimer P, Parizel P, at al. Clinical MR Imaging / A practical approach, page 38 ISBN 3-540-64098-3.
2 Tóth É, Helm L, Merbach A. Relaxivity of gadolinium(III) complexes: Theory and mechanism. In: Merbach A, Helm L, Toth E, eds. The chemistry of contrast agents in medical magnetic resonance imaging. Second Edition ed: John Wiley & Sons, Ltd; 2013:25 – 81.
3 Schild HH. MRI Made Easy... well almost [iOS App]. Version 1.5.1, Utrecht, The Netherlands: BestApps BV; 2018.
Gadovist® 1.0 mmol / mL solution for injection. Composition: 1 mL solution for injection contains 604.72 mg gadobutrol (equiv. 1.0 mmol gadobutrol containing
157.25 mg gadolinium) as active ingredient. Excipient with known effect: 1 mL contains 0.00056 mmol (equivalent to 0.013 mg) of sodium.
Indications: For diagnostic use only. Gadovist® is indicated in adults and children of all ages (including term neonates) for: 1.) Contrast enhancement in cranial and spinal magnetic resonance imaging (MRI); 2.) Contrast-enhanced MRI of liver or kidneys in patients with high suspicion or evidence of having focal lesions to classify these lesions as benign or malignant; 3.) Contrast enhancement in MR angiography; 4.) MR Imaging of pathologies of the whole body. Gadovist® facilitates visualisation of abnormal structures or lesions and helps in the differentiation between healthy and pathological tissue. Gadovist® should be used only when diagnostic information is essential and not available with unenhanced magnetic resonance imaging (MRI). Posology: Gadovist® should only be administered by healthcare professionals experienced in the field of clinical MRI practice. The lowest dose that provides sufficient enhancement for diagnostic purposes should be used. The dose should be calculated based on the patient’s body weight, and should not exceed the recommended dose per kilogram of body weight detailed in this section. Gadovist® is for intravenous administration only.
Contraindications: Hypersensitivity to the active substance or any of the excipients. Special warnings and precautions for use: While injecting Gadovist® into veins with a small lumen there is the possibility of adverse effects such as reddening and swelling. The usual safety requirements for MRI, especially the exclusion of ferromagnetic materials, also apply when using Gadovist®. Hypersensitivity reactions: As with other intravenous contrast agents, Gadovist® can be associated with anaphylactoid / hypersensitivity or other idiosyncratic reactions, characterized by cardiovascular, respiratory or cutaneous manifestations, and ranging to severe reactions including shock. In general, patients with cardiovascular disease are more susceptible to serious or even fatal outcomes of severe hypersensitivity reactions.
The risk of hypersensitivity reactions may be higher in case of 1.) previous reaction to contrast media; 2.) history of bronchial asthma; 3.) history of allergic disorders.
In patients with an allergic disposition the decision to use Gadovist® must be made after particularly careful evaluation of the risk-benefit ratio. Most of these reactions occur within half an hour of administration. Therefore, post-procedure observation of the patient is recommended. Medication for the treatment of hypersensitivity reactions as well as preparedness for institution of emergency measures are necessary. Delayed reactions (after hours up to several days) have been rarely observed.
Impaired renal function: Prior to administration of Gadovist® it is recommended that all patients are screened for renal dysfunction by obtaining laboratory tests. There have been reports of nephrogenic systemic fibrosis (NSF) associated with use of some gadolinium-containing contrast agents in patients with acute or chronic severe renal impairment (GFR < 30 mL / min / 1.73 m2). Patients undergoing liver transplantation are at particular risk since the incidence of acute renal failure is high in this group. As there is a possibility that NSF may occur with Gadovist®, it should therefore only be used in patients with severe renal impairment and in patients in the perioperative liver transplantation period after careful risk / benefit assessment and if the diagnostic information is essential and not available with non-contrast enhanced MRI. Haemodialysis shortly after Gadovist® administration may be useful at removing Gadovist® from the body. There is no evidence to support the initiation of haemodialysis for prevention or treatment of NSF in patients not already undergoing haemodialysis. Neonates and infants: Due to immature renal function in neonates up to 4 weeks of age and infants up to 1 year of age, Gadovist® should only be used in these patients after careful consideration. Elderly: As the renal clearance of Gadovist® may be impaired in the elderly, it is particularly important to screen patients aged 65 years and older for renal dysfunction. Seizure disorders: Like with other gadolinium containing contrast agents special precaution is necessary in patients with a low threshold for seizures. Pregnancy and lactation: There are no data from the use of Gadovist® in pregnant women. Gadovist® should not be used during pregnancy unless the clinical condition of the woman requires use of Gadovist®. Continuing or discontinuing of breast feeding for a period of 24 hours after administration of Gadovist®, should be at the discretion of the doctor and lactating mother.
Undesirable effects: The overall safety profile of Gadovist® is based on data from more than 6,300 patients in clinical trials and from post-marketing surveillance. The most frequently observed adverse drug reactions (≥ 0.5 %) in patients receiving Gadovist® are headache, nausea and dizziness. The most serious adverse drug reactions in patients receiving Gadovist® are cardiac arrest and severe anaphylactoid reactions (including respiratory arrest and anaphylactic shock). Delayed anaphylactoid reactions (hours later up to several days) have been rarely observed. Most of the undesirable effects were of mild to moderate intensity. Following adverse reactions have been observed: 1.) Common (≥ 1 / 100 to < 1 / 10) headache, nausea; 2.) Uncommon (≥ 1 / 1,000 to < 1 / 100) hypersensitivity/anaphylactoid reaction, dizziness, dysgeusia, paresthesia, dyspnea, vomiting, erythema, pruritus, rash, injection site reaction, feeling hot; 3.) Rare (≥ 1 / 10,000 to < 1 / 1,000) loss of consciousness, convulsion, parosmia, tachycardia, palpitations, dry mouth, malaise, feeling cold; 4.) Not known: cardiac arrest, NSF. Patients with an allergic disposition suffer more frequently than others from hypersensitivity reactions. Isolated cases of NSF have been reported with Gadovist®. Paediatric population: Frequency, type and severity of adverse reactions in children of all ages (including term neonates) are consistent with the adverse drug reaction profile known in adults.
Overdose: The maximum daily single dose tested in humans is 1.5 mmol gadobutrol / kg body weight. No signs of intoxication from an overdose have so far been reported during clinical use. In case of inadvertent overdosage, cardiovascular monitoring (including ECG) and control of renal function is recommended as a measure of precaution. In case of overdose in patients with renal insufficiency, Gadovist® can be removed by haemodialysis. After 3 haemodialysis sessions approx. 98 % of the agent are removed from the body.
However, there is no evidence that haemodialysis is suitable for prevention of NSF. Reporting of suspected adverse reactions: Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system or to DrugSafety.GPV.US@bayer.com.
Date of revision of text: December 2017.
Please note: For current prescribing information refer to the package insert and / or contact your local Bayer AG.


