

Primovist®
Primovist® is a liver-specific contrast agent for magnetic resonance imaging.
Liver. Specific. Confidence.
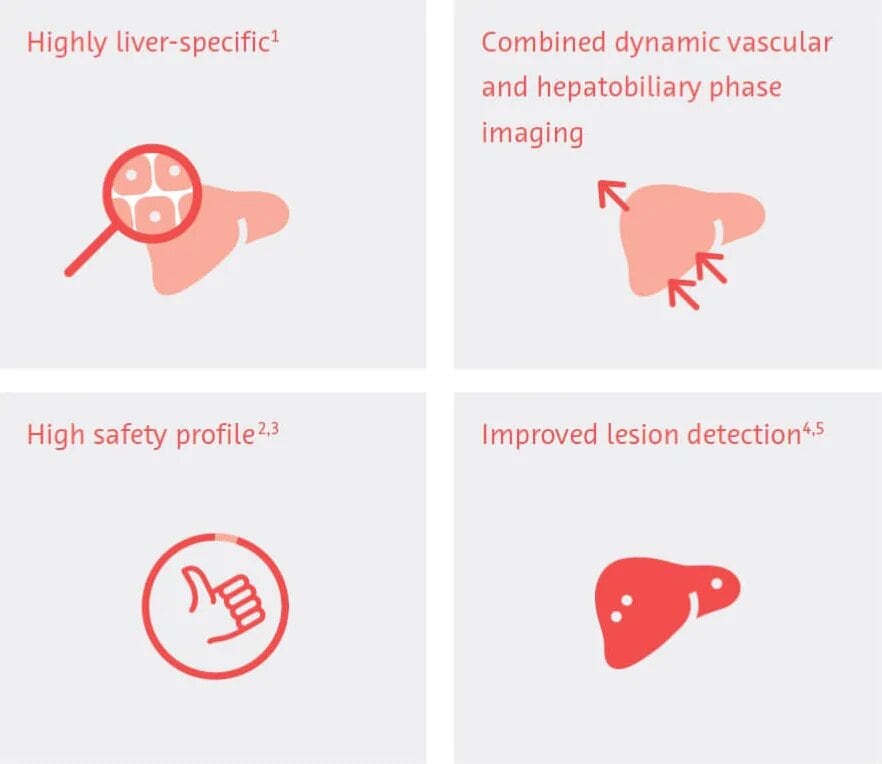
Primovist® significantly improves the detection and characterization of focal liver lesions and is particularly valuable in the detection of small lesions.1 Radiologists experience an increase in diagnostic confidence in the diagnosis of liver disease. After Primovist®-MRI no additional imaging procedure is required.2
What is Primovist®?
Primovist® is a 0.25 molar hepatocyte specific MRI contrast agent. It has combined properties of an extracellular agent and a liver specific agent.
How does Primovist® work?
The active ingredient of Primovist® is gadoxetate disodium. It is a gadolinium containing contrast agent in which the Gd3+ ion is firmly bound in a complex. Primovist® is first distributed in the extracellular space of the body, allowing traditional T1-weighted dynamic vascular images to be acquired. The vascular phase is followed by a gradual transition of the agent into hepatocytes through transporters within the cell membranes (transitional phase). The intracellular enhancement reaches a plateau about 10-20 minutes after Primovist® injection.
This accumulation of Primovist® in the liver cells allows an additional delayed imaging phase to take place with an extended imaging window. During this phase, healthy liver parenchyma is positively enhanced in T1-weighted images.
Application
Primovist® is provided in ready-to-use 10 mL prefilled glass syringes and in vials.
How is Primovist® administered?
Primovist® is administered intravenously as a fast bolus (1-2 mL/sec). Use of an injector is recommended and there is some evidence to suggest that a slower injection speed of 1 mL/sec may be beneficial, due to a reduction in imaging artifacts.
Indications
Primovist® is indicated for the detection of focal liver lesions and provides information on the character of lesions in T1-weighted MRI.6
1 Halavaara J et al. Comput Assist Tomogr. 2006;30:345–54.
2 Endrikat J et al. J Magn Reson Imaging. 2015;42(3):634–43.
3 Endrikat J et al. Acta Radiol. 2016; 57(11):1326–33.
4 Hammerstingl R, et al. Eur Radiol. 2008;18(3):457 – 467
5 Zech CJ, et al. Br J Surg. 2014;101(6):613–21.
6 Primovist® SmPC July 2016
.
Primovist® is:
- provided as a ready-to-use solution of 0.25 mmol Gd/mL.
- for diagnostic use by intravenous administration only.
- a stable colorless solution of low osmolality and low viscosity.
Physicochemical data
| Parameter | Gd-EOB-DTPA |
| Viscosity (at 37°C) | 1.19 mPa.s |
| Osmolality (at 37°C) | 688 mOsm/kg H2O |
| Density (at 37°C) | 1.0881 g/mL |
| Relaxivity r1 (at 1.5 Tesla, in plasma, 37°C) | 6.9 l/(mmol-1s-1) [Rohrer et al.] |
| Relaxivity r2 (at 1.5 Tesla, in plasma, 37°C) | 8.7 l/(mmol-1s-1) [Rohrer et al.] |
Primovist® administration at 25 µmol/kg BW provided improved diagnostic confidence in 90% of patients in phase 2 studies.
Primovist® is supplied in ready-to-use, prefilled 10 mL syringes containing 10 mL (1814 mg Gd-EOB-DTPA) of solution.
Primovist® 0.25 mmol / mL, solution for injection, prefilled syringe (gadoxetate disodium). Prescribing Information (Refer to Full Summary of Product Characteristics (SmPC) before prescribing).
Presentation: Each mL solution for injection contains 181.43 mg / mL gadoxetate disodium.
Indication: Detection of focal liver lesions and providing information on the character of lesions in T1-weighted magnetic resonance imaging (MRI).
Posology and method of administration: Primovist® should be used only when diagnostic information is essential and not available with unenhanced MRI and when delayed phase imaging is required. Observe usual safety precautions for MRI (e.g. exclude cardiac pacemakers and ferromagnetic implants). Use lowest dose that provides sufficient enhancement for diagnostic purposes calculated based on the patient’s body weight, and do not exceed the recommended dose. Administer dose undiluted as an intravenous bolus injection at a flow rate of about 2 mL / sec. After injection, flush cannula / line with 0.9 % saline. Observe patients for at least 30 minutes after the injection.
Recommended doses are:
Adults: 0.1 mL / kg body weight. Impaired renal function: Use of Primovist® should be avoided in patients with severe renal impairment (GFR < 30 mL / min / 1.73 m2) and in patients in the perioperative liver transplantation period unless the diagnostic information is essential and not available with non-contrast enhanced MRI. If use cannot be avoided, dose should not exceed 0.025 mmol / kg body weight. Do not use more than one dose per scan. Do not repeat the dose for at least 7 days.
Patients with hepatic impairment: No dose adjustment necessary.
Paediatric population: The safety and efficacy of Primovist® have not been established in patients under 18 years old. However, an observational study was performed in 52 paediatric patients (aged > 2 months and < 18 years). Patients were referred for Primovist® contrast-enhanced liver MRI to evaluate suspected or known focal liver lesions. Additional diagnostic information was obtained when combined unenhanced and enhanced liver MR images were compared with unenhanced MR images alone. Serious adverse events were reported, however none were assessed by the investigator to be related to Primovist®. Due to the retrospective nature and small sample size of this study, no definitive conclusion can be made regarding efficacy and safety in this population. No dose adjustment necessary. Elderly population (≥ 65 years): No dose adjustment necessary. Exercise caution.
Accumulation in the body: After administration of Primovist® gadolinium (Gd) can be retained in the brain and in other tissues of the body and can cause dose-dependent increases in T1w signal intensity in the brain, particularly in the dentate nucleus, globus pallidus, and thalamus. Signal intensity increases and non-clinical data show that Gd is released from linear GBCAs. Clinical consequences are unknown. The possible diagnostic advantages of using Primovist® in patients who will require repeated scans should be weighed against the potential for deposition of Gd in the brain and other tissues.
Contraindications: Hypersensitivity to active substance or to any excipients.
Warnings and precautions: It is recommended to screen all patients for renal dysfunction by obtaining laboratory tests, particularly patients over 65 years. Nephrogenic systemic fibrosis (NSF) has been reported with some gadolinium-containing contrast agents in patients with acute or chronic severe renal impairment (GFR < 30 mL / min / 1.73 m2); Patients undergoing liver transplantation are at particular risk since incidence of acute renal failure is high in this group. Use should be avoided in patients with severe renal impairment and in patients in perioperative liver transplantation period unless diagnostic information is essential and not available with non-contrast enhanced MRI. Haemodialysis shortly after Primovist® administration may be useful at removing Primovist® from the body. There is no evidence to support initiation of haemodialysis for prevention or treatment of NSF in patients not already undergoing haemodialysis.
Use with caution in patients: with severe cardiovascular problems; with, or with a family history of, congenital long QT syndrome; with drugs known to prolong cardiac repolarisation, particularly in patients with previous arrhythmias. Should not be used in patients with uncorrected hypokalaemia. Primovist® may cause transient QT prolongation. Allergy-like reactions, including shock, reported rarely. Patients with a history of allergic disorders or bronchial asthma or who have previously reacted to contrast media are at higher risk of hypersensitivity reactions. Most reactions occur within 30 minutes of administration but rarely delayed reactions may occur after hours to days. Appropriate drugs and instruments for treatment of hypersensitivity must be readily available. Hypersensitivity reactions can be more intense in patients on beta-blockers, particularly in patients with asthma. Patients taking beta-blockers who experience hypersensitivity may be resistant to treatment effects of beta-agonists. If hypersensitivity reactions occur, stop injection immediately. Do not administer intramuscularly due to risk of local intolerance reactions including focal necrosis. Consider the sodium content (11.7 mg / mL) for patients on controlled sodium diet.
Interactions: Potent OATP inhibitors could cause drug interactions reducing the hepatic contrast effect. No clinical data exists to support this theory. Elevated levels of bilirubin or ferritin can reduce the hepatic contrast effect of Primovist®. Primovist® may interfere with serum iron determinations for up to 24 hours after administration.
Pregnancy and lactation: There are no data from use in pregnant women. Animal studies have shown reproductive toxicity at repeated high doses. Should not be used in pregnancy unless clinical condition of the woman requires the use of Primovist®. Gd-containing contrast agents are excreted into breast milk in very small amounts. Continuing or discontinuing breast feeding for 24 hours after administration should be at discretion of the doctor and lactating mother.
Undesirable effects: (please refer to the Contraindications and the Warnings and Precautions sections). Usually mild to moderate and transient. The most serious adverse reaction is anaphylactoid shock. Delayed allergoid reactions (hours later up to several days) are rare. Common: Headache, nausea. Uncommon: Vertigo, dizziness, dysgeusia, paraesthesia, parosmia, increased blood pressure, flushing, dyspnoea, respiratory distress, vomiting, dry mouth, rash, pruritus, back pain, chest pain, injection site reactions, feeling hot, chills fatigue. Rare: Tremor, akathisia, bundle branch block, palpitation, maculopapular rash, hyperhidrosis, malaise. Additionally, altered laboratory tests and transient QT prolongation were reported. Frequency not known: Hypersensitivity/anaphylactoid reaction (including shock*, hypotension, pharyngolaryngeal oedema, urticaria, face edema, rhinitis, conjunctivitis, abdominal pain, hypoesthesia, sneezing, cough, pallor), tachycardia and restlessness.*Lifethreatening and / or fatal cases have been reported post marketing. Prescribers should consult the SmPC in relation to other side effects.
Overdose: In excessive inadvertent overdose, monitor patient including cardiac monitoring (for possible induction of QT prolongation); remove by haemodialysis. However there is no evidence that haemodialysis is suitable for prevention of nephrogenic systemic fibrosis (NSF).
Reporting of suspected adverse reactions: Adverse events can be reported to
DrugSafety. GPV.US@bayer.com.
Date of revision text: December 2017.
Please note: For current prescribing information refer to the package insert and / or contact your local Bayer AG.
Studies
Validation of DCE-MRI for Liver Malignancies
Perfusion parameters of dynamic Primovist®-enhanced MRI correlate with the proliferation and...
News on Benign Liver Lesions
Differentiation between the benign liver lesion focal nodular hyperplasia (FNH) and hepatocellular...
Primovist® in Korean Guidelines
A Korean study confirms the advantages of having included specific features of HCC...
Primovist® in the Elderly
Gadoxetate disodium shows a good safety profile in patients older than 65 years...
Interviews

"Radiologists should stand up for themselves"
Takamichi Murakami is Professor and Chairman of the Department of Radiology at Kinki University Faculty of Medicine in Osaka, Japan.
The Japanese way of managing HCC
Professor Masayuki Kanematsu, Gifu University Hospital, is one of the leading Japanese liver imaging specialists.

Disclaimer
Effective November 23, 2017, the European Commission (EC) issued a final decision in the Article 31 referral procedure evaluating the presence of gadolinium (Gd) in the body and gadolinium-based contrast agents (GBCAs) to the EU Member States and countries in the European Economic Area (EEA). In general, the EC has adopted the opinion of the European Medicine’s Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP). The EC decision is two-fold:
- Suspension of all multipurpose linear GBCAs, including Bayer’s Magnevist® i.v.
- Updates to the labels / prescribing information of all GBCAs remaining on the market, including Bayer’s Gadovist® 1.0 and its specialty linear GBCAs Primovist® and Magnevist® 2 mmol / L intra-articular formulation.
Importantly, EMA’s CHMP in their final opinion confirms that “there is currently no evidence that gadolinium deposition in the brain has caused any harm to patients; however EMA has recommended restrictions for some intravenous linear agents in order to prevent any risk that could potentially be associated with gadolinium brain deposition.”
Magnevist® is not available in all countries.


